Recently, Nucleic Acid Extraction and Purification Kit independently developed and produced by igenesis has obtained IVDR-CE certification, which indicates that the igenesis’s product quality has been recognized and affirmed by international authorities, and igenesis has obtained access to the EU market, laying a solid foundation for the continued sales of in the EU!


CE certification and IVDR
CE certification is regarded as a "visa" for products to enter the European market, and is a mandatory requirement for product access in the EU market.
From 26th, May, 2022, the European Union officially implemented the in vitro diagnostic medical device Regulation EU 2017/746 (IVDR), in vitro diagnostic medical devices that have not obtained IVDR CE certification cannot enter the EU market.
The new regulations aim to establish a more modern and stringent regulatory framework, in order to better protect the health and safety of the public and patients.The new Regulation IVDR has been upgraded from Directive to Regulation. Compared with the IVDD Directive, IVDR puts forward more stringent requirements for the manufacturer's quality system and the safety, effectiveness and post-market supervision of products, and requires products to meet the traceability from production to the end.
Nucleic Acid Extraction and Purification Kit
The lgenesis Nucleic Acid Extraction and Purification Kit is designed to provide a diverse range of molecular point-of-care testing solutions, which is compatible with the third-party detection reagents to supply more business possibilities.
Product Advantages
● Magnetic beads nucleic acid extraction technology
● Accurate results: CV value ≤5%, coincidence rate is 100%
● Extract nucleic acids from complex biological samples (swab,serum,plasma,urine,sputum,saliva,tissue,etc.)
● Solid material transfer+Microfluidic technology
● Preloading reagent+Patented freeze-drying technology
● Fully enclosed iCassette, Prevent pollution, Ensure biosafety
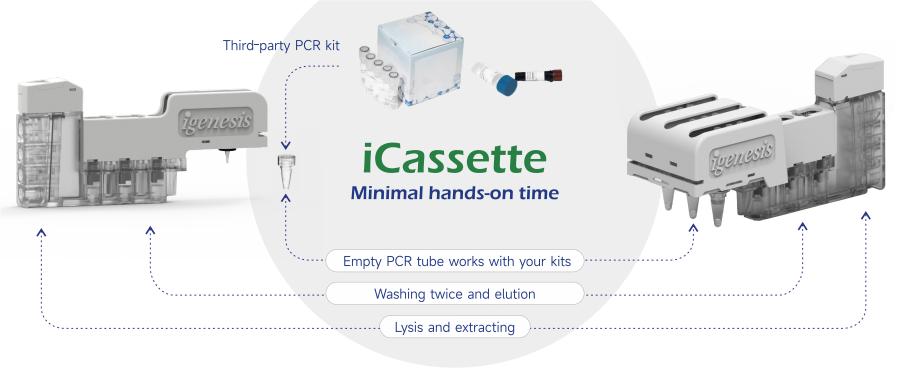
Compatibility and flexibility:
● Adaptation of third-party PCR reagents
● Customized collaboration mode
● 7-colour fluorescent system, Up to 54 multiple targets
At present, igenesis has obtained IVDR-CE mark for 6 products,overseas business is distributed globally, such as Italy, Chile, Malaysia, Spain, Denmark, Hong Kong, China and Europe, South America, Southeast Asia and other regions.In the future, igenesis will always adhere to the corporate philosophy of "integrity, excellence, innovation and win-win",which provide safe, fast, convenient and accurate POCT solutions for our customers. Empowering Nucleic Acid Testing and boosting POCT of nucleic acid testing reagents.
Post time: Sep-01-2023
