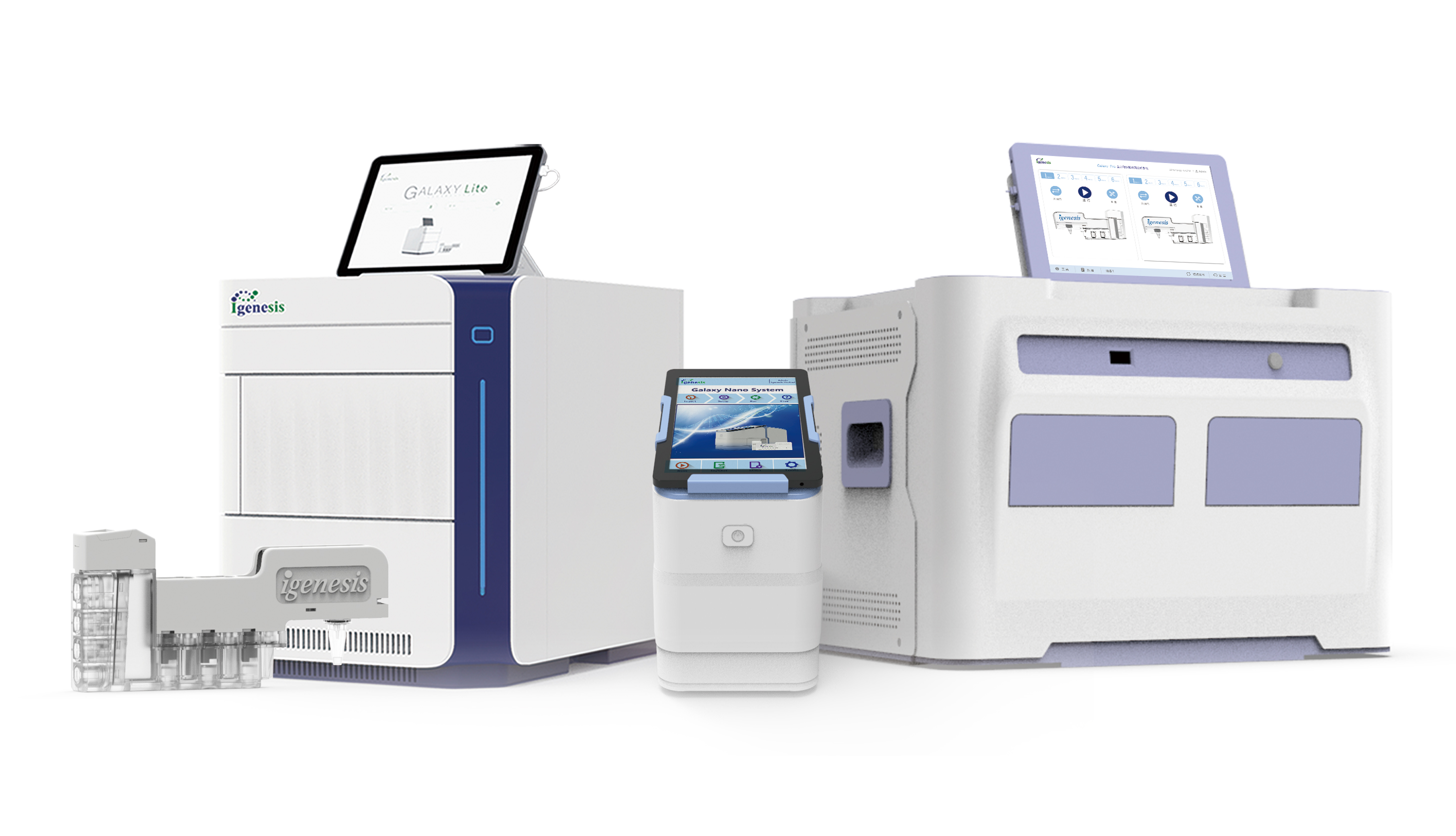
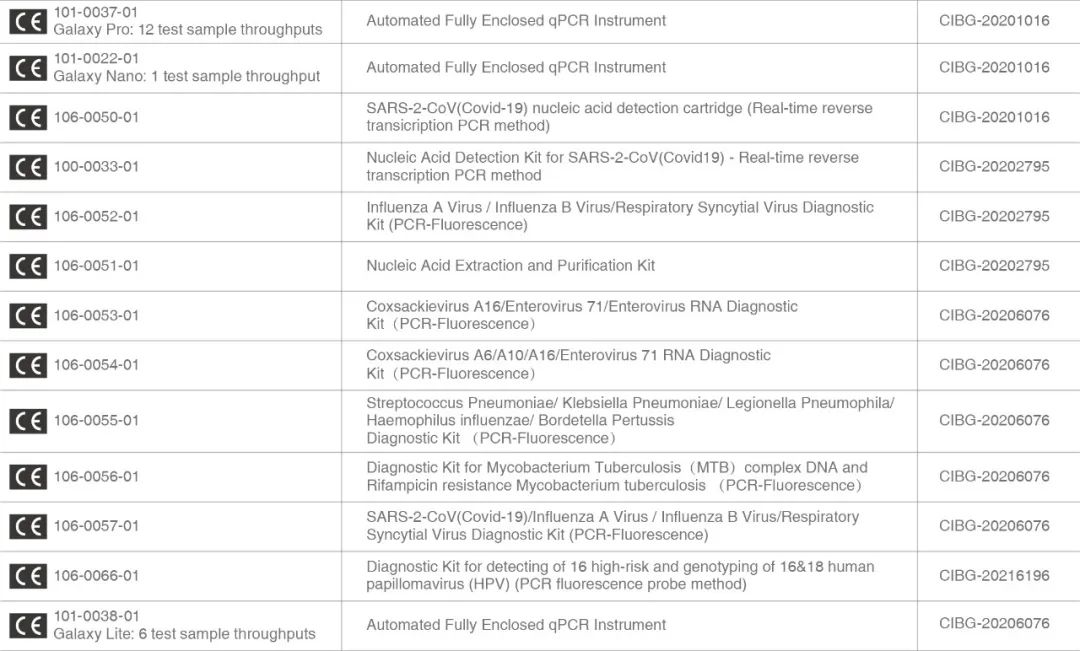
Recently, Igenesis’s iCassette diagnostics kit for detecting type 16 human papillomavirus (HPV) and genotyping type 16 and type 18 HPV using PCR fluorescence probes obtained the EU CE certification. As of December 2021, that would make a total of 13 products that have been EU CE certified, including Igenesis’s Automated Fully Enclosed qPCR Instruments (the Galaxy Nano, Galaxy Lite, and Galaxy Pro) and other varieties of our iCassettes (see the product list at the bottom for details).


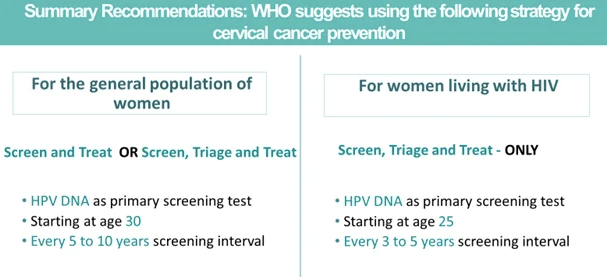
New Recommendation from the WHO for HPV DNA Primary Screening Test
On July 6, 2021, the World Health Organization (WHO) issued their 2nd edition guidelines for the screening and treatment of cervical pre-cancerous lesions for cervical cancer prevention. The guideline recommended HPV DNA as the primary screening test to promote cervical cancer prevention and save more lives.
Fast and Accurate Screening is Essential for Prevention, Early Detection and Early Treatment of Cervical Cancer
Igenesis’s POCT molecular diagnostic platform for HPV DNA detection has broken through previous PCR lab restrictions. Using classic magnetic bead extraction and fluorescent PCR technology for accurate HPV DNA testing in a broader range of scenarios will improve the efficiency of cervical cancer screening and contribute to the prevention and mitigation of cervical cancer.
For Cervical Cancer
Cervical cancer is a Global Public Health Problem
In 2020, an estimated 604,000 women were diagnosed with cervical cancer worldwide, and about 342,000 women died from the disease. In November 2020, the WHO launched their global strategy to accelerate the elimination of cervical cancer with the goal of achieving the following by 2030: a 90% human papillomavirus (HPV) vaccination coverage of eligible women, a 70% screening coverage with a high-performance test, and 90% of women with a positive screening test result or a cervical lesion(s) treated appropriately.
Cervical Cancer is a Preventable and Treatable Disease
Cervical cancer is currently the only cancer with a clear cause; high-risk HPV is the cause in nearly all cases (99%). Among them, 70% of cases are caused by only two types of HPV (type 16 and type 18), both of which appear as common viral infections transmitted through sexual, skin-to-skin contact.
Almost all women will contract HPV at some point in their lives, and most will have their immune systems eliminate the virus on their own without any help. However, for some women, the infection persists and can progress into cervical pre-cancer or cancer with enough time.
HPV screening and vaccination are important means of cervical cancer prevention. Receiving the HPV vaccination can prevent infection of different HPV subtypes and cervical intraepithelial neoplasia (CIN) for the general population. Through HPV screening, cervical pre-cancerous lesions can be detected early, so as to carry out effective treatment and management.
Cervical cancer is one of the cancers that may be successfully treated. With a comprehensive approach to preventing, screening, and treating cervical cancer, the disease can be expected to be eliminated within a generation.
Igenesis’s CE product catalog:
Post time: Dec-13-2021
