The 34th European Congress of Clinical Microbiology and Infectious Diseases (ESCMID Global 2024) concluded in Barcelona, Spain, from April 27 to 30, 2024. ESCMID Global is recognized as the largest and most comprehensive global academic conference in the fields of clinical microbiology, infectious diseases, and infection control. The congress, organized by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), brought together international experts from various domains to share their latest discoveries, guidelines, and experiences.

During this event, Igenesis made a stunning appearance at booth D35-2, showcasing its innovative spirit and technical prowess. They presented the Galaxy Automated Fully Enclosed qPCR System, along with a range of reagents developed in-house. The booth attracted a steady stream of visitors, including global experts, scholars, company representatives, and exhibition attendees.
Igenesis’s overseas marketing team vividly demonstrated the overall solution and technological innovations in the field of point-of-care molecular diagnostics (POCT). The audience praised their products for their unique design concepts, outstanding performance, and versatile applications. The lively atmosphere on-site prompted many customers to express interest in collaboration and procurement.
Exhibition highlights






Galaxy Automated Fully Enclosed qPCR System
The Galaxy Automated Fully Enclosed qPCR System, simplifies the workflow and enhances testing efficiency. Paired with the iCassette (PCR-fluorescent probe method), it completes the entire process of nucleic acid extraction, purification, and fluorescent PCR amplification in just three steps, all in a fully enclosed manner. The system supports one-click testing for multiple projects, including HPV screening, STD testing, respiratory virus panel testing, tuberculosis screening, drug resistance testing, and more, catering to diverse testing needs.
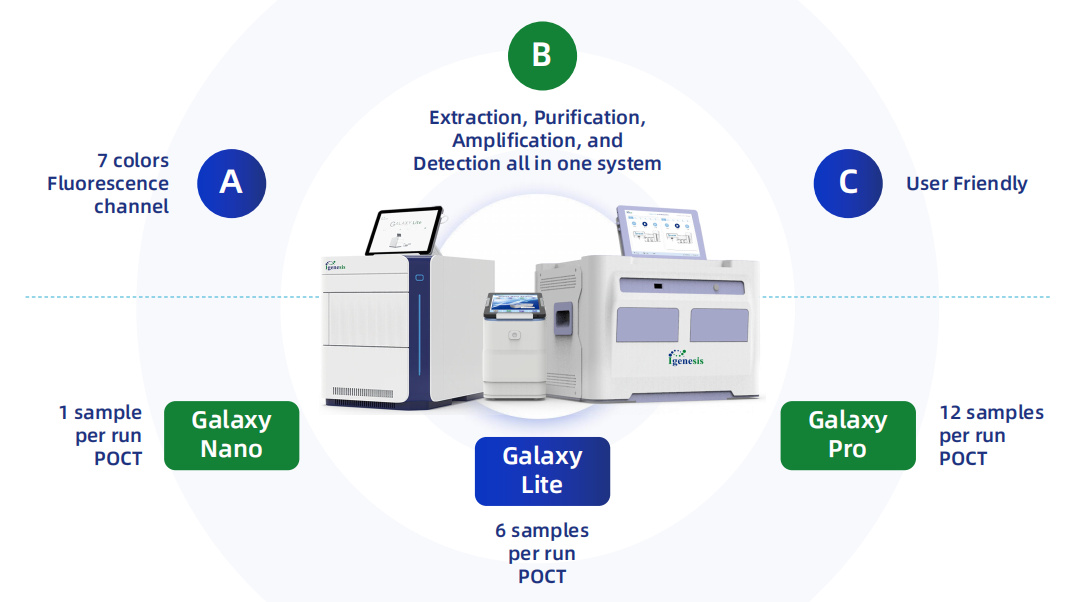
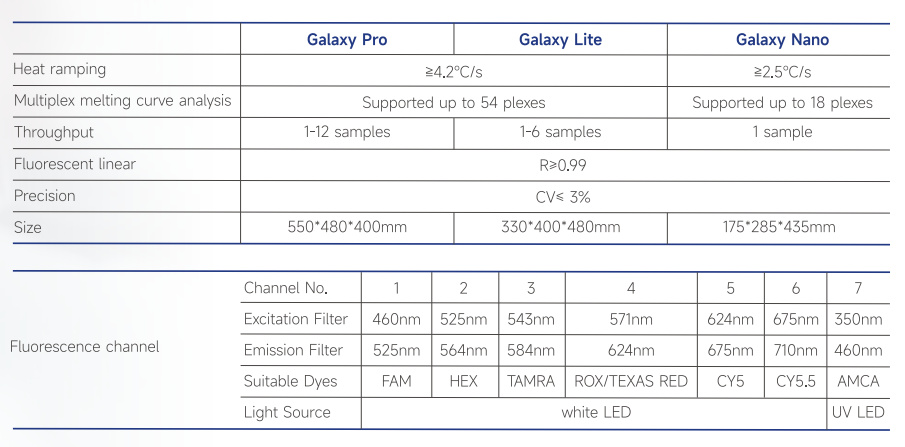
iCassette®
The iCassette, as an open platform, has revolutionized the field of molecular diagnostics with its compatibility and flexibility. It integrates third-party traditional fluorescent PCR test reagents into the iCassette, creating an all-in-one molecular point-of-care testing (POCT) product. When used in conjunction with the Galaxy Automated Fully Enclosed qPCR System, it achieves a fully enclosed, integrated workflow for nucleic acid testing. This not only significantly saves product development time and costs but also enables rapid pathogen nucleic acid testing in diverse settings such as healthcare institutions, disease control centers, third-party inspection agencies, forensic centers, and quarantine bureaus.

About Igenesis
Igenesis’s technical strength and innovation capabilities have been widely recognized both domestically and internationally. As of now, Igenesis has obtained more than 120 domestic and foreign intellectual property patents, 9 Chinese medical device registration certificates, and 21 EU CE certifications. In the future, Igenesis remains committed to its core values of integrity, excellence, innovation, and collaborative success. The company will continue to explore the limitless potential of genetic testing, providing customers with secure, rapid, convenient, and precise integrated molecular diagnostic solutions.

Post time: Aug-13-2024
