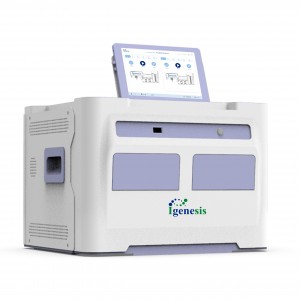
Galaxy Lite
Features
1.Intelligent
Automatically complete the whole process of fluorescence qPCR, reducing operator requirements.
2. Integrated
Extraction+amplification+detection 3-in-1, no need for additional equipment investment.
3. Fully enclosed
Eliminate contamination, ensure biosafety, no need to partition the PCR lab.
4. Modularity
6 samples/single module, up to 4 modules cascade, more flexible throughput.
Minimal Hands-On Time 3 simple steps to get qPCR results

Load the sample into the iCassette

Put the iCassette into the instrument

Auto run to receive qPCR results
Instrument Performance
|
Galaxy Lite |
||
|
Intelligent |
Automated nucleic acid extraction and purification | Yes |
| Automated transfer of nucleic acid to the amplification area | Yes | |
| Automated protocol programming | Yes | |
| Automated qPCR results analysis | Yes | |
| Fluorescence channel | 7-color fluorescence channel, up to 6-fold fluorescence detection: Fam, Hex, Tamra, Rox, Cy5, Cy5.5, Amca | |
| Fluorescence linear | R≥0.99 | |
| Precision | CV≤3% | |
| Size (mm) | 327*405*473mm | |
The Galaxy Lite can be upgrated to run 24 samples in parallel, 4 protocols asynchronous
Samples
Galaxy Lite Module Combination


Making Nucleic Acid Detection Safer·Faster·Easier·More Accurate
iCassette——Preloaded Reagent
Ready for Use

● Magnetic bead transfer structure and process technology (Invention Patent)
● Material distributed mixing device structure and technology (Invention Patent)
● Solid material transfer + microfluidic technology
● Fully enclosed, fully automatic, no need for manual operation
● Extraction of proteins and nucleic acids from complex biological samples
● Preloaded reagent, patented freeze-drying technology
● No need for freezer storage or transportation
● Internal control is involved in the whole process of the experiment to avoid false negative results
Application
| Classification | Product Name | Remark |
| Respiratory Infectious Diseases | SARS-2-CoV(Covid-19) nucleic acid detection cartridge (Real-time reverse transicription PCR method) | 3-plexes Detection(with internal control) |
| SARS-2-CoV(Covid-19)/Influenza A Virus / Influenza B Virus/Respiratory Syncytial Virus Diagnostic Kit (PCR-Fluorescence) | 6-plexes Detection(with internal control) | |
| Flu A/FluA H1/FluA H3 /FluB Victoria/FluB Yamagata Diagnostic Kit (Real-time PCR) | 6-plexes Detection(with internal control) | |
| RSV A/RSV B Diagnostic Kit (Real-time PCR) | 3-plexes Detection(with internal control) | |
| Diagnostic Kit for Mycobacterium Tuberculosis(MTB)complex DNA and Rifampicin resistance Mycobacterium tuberculos (PCR-Fluorescence) | 7-plexes Detection(with internal control) | |
| Influenza A Virus / Influenza B Virus/Respiratory Syncytial Virus Diagnostic Kit (PCR-Fluorescence) | 4-plexes Detection(with internal control) | |
| MP/CP/AdV Diagnostic Kit (Real-time PCR) | 4-plexes Detection(with internal control) | |
| Streptococcus Pneumoniae/ Klebsiella Pneumoniae/ Legionella Pneumophila/ Haemophilus influenzae/ Bordetella Pertussis Diagnostic Kit (PCR-Fluorescence) | 6-plexes Detection(with internal control) | |
| Intestinal Infectious Diseases | Coxsackievirus A6/A10/A16/Enterovirus 71 RNA Diagnostic Kit(PCR-Fluorescence) | 5-plexes Detection(with internal control) |
| Coxsackievirus A16/Enterovirus 71/Enterovirus RNA Diagnostic Kit(PCR-Fluorescence) | 4-plexes Detection(with internal control) | |
| Sexual Infectious Diseases | CT/NG/UU Diagnostic Kit (Real-time PCR) | 4-plexes Detection(with internal control) |
| Diagnostic Kit for detecting of 16 high-risk and genotyping of 16&18 human papillomavirus (HPV) (PCR fluorescence probe method) | 6-plexes Detection(with internal control) | |
| Arbo Infectious Diseases | DENV I/DENV II/DENV III/DENV IV Diagnostic Kit (Real-time PCR) | 5-plexes Detection(with internal control) |
| DENV/CHIKV/ZIKV Diagnostic Kit (Real-time PCR) | 4-plexes Detection(with internal control) | |
| Universal kit | Galaxy CS EXT Nucleic Acid Extraction and Purification Kit | Compatible with 3rd party reagents |
System Components